Imprints: Bacterial Photography, By Jeff Tabor
Bacterial Photography, By Jeff Tabor
IMPRINTS |FALL/WINTER 2010
In 2005, a research group from the University of California at San Francisco and the University of Texas at Austin published a report in Nature describing the engineering of the bacterium Escherichia coli (E. coli) to function as a black and white photographic film. Unlike their virulent cousins, these laboratory bacteria are completely non-pathogenic. Over time, bacterial films have been assigned a variety of monikers ranging from “Coliroids€ to “E.guerrotypes.€
The construction of a living photographic film required two main technological advances. The otherwise blind E. coli first were genetically engineered to see red light and produce a visible black pigment. Second, a ‘bacterial camera’ supporting the growth of the photographic cells was constructed. When the engineered E. coli are grown in the dark, the light sensor is fully active, resulting in high-level accumulation of black pigment. When the bacteria are exposed to saturating intensities of red light, the sensor becomes inactive, resulting in about 10 times less black pigment production per bacterial cell. Intermediate levels of red light result in intermediate levels of black pigment over a large range of light intensities, allowing faithful greyscales to be produced (see figure).
The bacterial films respond to as little as ~0.002W/m2 650nm filtered light. As a practical measure this is just enough light to detect with the naked eye. The films saturate at ~0.04W/m2 650nm light, an intensity that appears very vibrant to an observer.
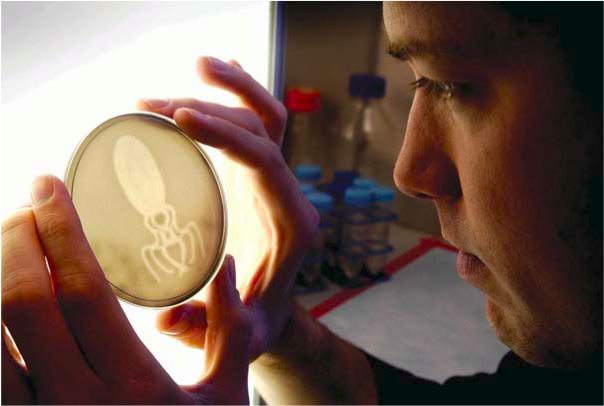
To produce bacterial photographs, approximately 1 million engineered E. coli are mixed into a warm molten solution of growth media. This solution is cast into a common Petri Dish and set at room temperature to solidify into a slab (or film) with the consistency of a gelatin dessert. The slab is then placed inside an incubator (a warm black box) and a red-filtered Kodak projector is used to shine a focused image of light onto the surface of the slab (http://openwetware.org/wiki/LightCannon). The bacteria are grown for approximately 12 hours, during which time the number of bacteria increases to approximately 1 billion. The bacteria do not move or swim within the slab, but rather grow into stationary clumps. Like a traditional film, the bacterial pixels are fixed; any bacterium exposed to dark at the beginning of an experiment remains in the dark for the entirety
At the end of the 12-hour exposure, the bacterial slab is removed from the incubator and a positive print of the image can be seen clearly by the naked eye (see figure). The slabs are wrapped in plastic to prevent drying and can be stored in a refrigerator for many months without a loss of sample quality. Eventually the slabs dry out, resulting in a very thin flaky film, but the image remains. Some images have been kept for over four years and retain the same sharpness and contrast as the moment the light exposure was finished. The slabs have also been removed from the Petri Dishes and encased within epoxy resins. These treated slabs do not dry out and appear to be stable indefinitely. Due to the nature of bacterial growth (and death), there is a developmental window during which the films are sensitive to light. After the films yield sufficient contrast to be seen, they do not appreciably change, even if re-exposed to light. In this way, bacterial films function much like Polaroid.
Since 2005, the original bacterial film technology has been advanced upon. First, several information processing ‘genetic circuits’ were placed between the red light sensor and the pigment producing enzyme in order to program the film to function as a bacterial ‘edge detector.’ Here the bacteria do not print positives of an applied image but trace the outlines of shapes within the image (http://www.microbialart.com/galleries/jeff-tabor/). Sensors responding to different colors of light have also been engineered recently. These are being linked to the production of different colored pigments in the media in order to expand bacterial photography to a color technology.
Jeff Tabor
Assistant Professor
Rice University, Department of Bioengineering